Background
Zirconium, symbol Zr on the Periodic Table, is a metal most often found in and extracted from the silicate mineral zirconium silicate and the oxide mineral baddeleyite. In its various compound forms, the grayish-white zirconium is the nineteenth most plentiful element in the earth's crust, where it is far more abundant than copper and lead. It belongs to the titanium family of metals, a group that also includes titanium and hafnium and that is favored in industry for its members' good electrical conductivity as well as their tendency to form metallic salts. Because it is stable in many electron configurations and physical states, zirconium can be made into many products. However, since the 1940s, its most significant applications have been in various structural components of nuclear reactors.
Zirconium was discovered by German chemist Martin Heinrich Klaproth, who first isolated an oxide of the mineral zircon in 1789. The first metallic powder was produced in 1824 by a Swedish Chemist, Jons J. Berzelius. The forms of the metal that could be isolated during the nineteenth century, however, were impure and thus very brittle. The earliest method of purifying useable quantities of the metal was developed in 1925 by Dutch chemists Anton E. van Arkel and J. H. de Boer, who invented a thermal iodide process by which they thermally decomposed zirconium tetraiodide. The drawback with van Arkel and de Boer's method was its cost, but twenty years later William Justin Kroll of Luxembourg invented a cheaper process, using magnesium to break down zirconium tetrachloride. Relatively inexpensive, this process produced zirconium in quantities large and pure enough for industrial use.
Since Kroll's breakthrough, zirconium has become an important element in several industries: steel, iron, and nuclear power. It is used in the steel industry to remove nitrogen and sulfur from iron, thereby enhancing the metallurgical quality of the steel. When added to iron to create an alloy, zirconium improves iron's machinability, toughness, and ductility. Other common industrial applications of zirconium include the manufacture of photoflash bulbs and surgical equipment, and the tanning of leather.
Despite its ability to be used for many different industrial applications, most of the zirconium produced today is used in water-cooled nuclear reactors. Zirconium has strong corrosion-resistance properties as well as the ability to confine fission fragments and neutrons so that thermal or slow neutrons are not absorbed and wasted, thus improving the efficiency of the nuclear reactor. In fact, about 90 percent of the zirconium produced in 1989 was used in nuclear reactors, either in fuel containers or nuclear product casings.
Raw Materials
Of the two mineral forms in which zirconium occurs, zircon is by far the more important source. Found mainly in igneous rock, zircon also appears in the gravel and sand produced as igneous rock erodes. In this form, it is often mixed with silica, ilmenite, and rutile. The vast majority of the zircon used in industry today originates in these sand and gravel deposits, from which the purest zircon is extracted and refined to be used as zirconium metals. Less pure deposits are used in the form of stabilized zirconia for refractories and ceramic products. The world's largest zircon mines are in Australia, South Africa,
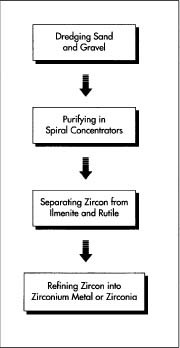
The sand and gravel that contain zircon are typically collected from coastal waters by a floating dredge, a large steam shovel fitted on a floating barge. After the shovel has scooped up the gravel and sand, they are purified by means of spiral concentrators, and then unwanted material is removed by magnetic and electrostatic separators.
End-product manufacturers of zircon further refine the nearly pure zircon into zirconium by using chlorine to purify the metal and then sintering (heating) it until it becomes sufficiently workable for industrial use. Less-pure zircon is made into zirconia, an oxide of zirconium, by fusing the zircon with coke, iron borings, and lime until the silica is reduced to silicon that alloys with the iron.
End-product manufacturers of zircon further refine the nearly pure zircon into zirconium by using chlorine to purify the metal and then sintering (heating) it until it becomes sufficiently workable for industrial use. Less-pure zircon is made into zirconia, an oxide of zirconium, by fusing the zircon with coke, iron borings, and lime until the silica is reduced to silicon that alloys with the iron.
Extraction and Refining
Extracting zircon
- 1 The sand and gravel that contain zircon mixed with silicate, ilmenite, and rutile are typically collected from coastal waters by a floating dredge, a large steam shovel fitted on a floating barge. After the shovel has scooped up the gravel and sand, they are purified by means of spiral concentrators, which separate on the basis of density. The ilmenite and rutile are then removed by magnetic and electrostatic separators. The purest concentrates of zircon are shipped to end-product manufacturers to be used in metal production, while less pure concentrations are used for refractories.
Refining zircon
- 2 End-product manufacturers of zircon further refine the nearly pure zircon into zirconium by using a reducing agent (usually chlorine) to purify the metal and then sintering (heating) it until it becomes sufficiently ductile—workable—for industrial use. For small-scale laboratory use, zirconium metal may be produced by means of a chemical reaction in which chloride is used to reduce the zircon.
- 3 The less-pure zircon is made into zirconia, an oxide of zirconium, by fusing the zircon with coke, iron borings, and lime until the silica is reduced to silicon that alloys with the iron. The zirconia is then stabilized by heating it to about 3,095 degrees Fahrenheit (1,700 degrees Celsius), with additions of lime and magnesia totalling about five percent.
Refining baddeleyite
- 4 As mentioned above, baddeleyite contains relatively high, pure concentrations of zirconium oxide that can be used without filtering or cleansing. The only refining process used on baddeleyite involves grinding the gravel or sand to a powder and sizing the powder with different sized sieves. All zirconium oxide that comes from baddeleyite is used for refractories and, increasingly, advanced ceramics.
Quality Control
The quality control methods implemented in the production of zirconium metal are typical Statistical Process Control (SPC) methods used in most metal production. These involve tracking and controlling specific variables determined by the end product requirements. Stringent government quality control is applied to all zirconium metal produced for nuclear applications. These controls assure that the zirconium produced for use in a nuclear plant has been processed correctly and also allow for accountability: processing is tracked so that it can be traced back to each individual step and location.
Quality control methods for zirconium used in refractory applications also focus on SPC. However, in the refractory industries, it is also necessary to ascertain the beach (and even what part of the beach) from which the zirconium mineral was extracted. Manufacturers need to know exactly where the zirconium came from because each source contains slightly different trace elements, and different trace elements can affect the end product.
Byproducts/Waste
Silicate, ilmenite, and rutile—all byproducts of the zircon refining process—are typically dumped back in the water at the extraction site. These elements compose typical beach sand and are in no way detrimental to the environment. Magnesium chloride, the only other notable byproduct of zirconium manufacturing, results from the reduction of the zircon with chlorine in the refining process and is typically sold to magnesium refineries. No byproducts or waste result from baddeleyite refining.
The Future
Many believe that the future of zirconium lies in its use as an advanced ceramic. Advanced ceramics—also called "fine," "new," "high-tech," or "high-performance" ceramics—are generally used as components in processing equipment, devices, or machines because they can perform many functions better than competing metals or polymers. Zirconium is fairly hard, doesn't conduct heat well, and is relatively inert (i.e., it doesn't react readily with other elements), all excellent qualities for advanced ceramics. Zirconium oxide, manufactured as a ceramic, can be used to make crucibles for melting metals, gas turbines, liners for jet and rocket motor tubes, resistance furnaces, ultra-high frequency furnaces, and refractories such as the facing of a high-temperature furnace wall.
Where To Learn More
Books
Heuer, A. H., ed. Science and Technology of Zirconia. American Ceramic Society, 1981.Specifications for Zirconium and Zirconium Alloy Welding Electrodes and Rods. American Welding Society, 1990.
Zirconium and Hafnium. Gordon Press Publishers, 1993.
Periodicals
Burke, Marshall A. "Ceramics Enter the Foundry," Design News. June 16,1986, p. 56."Fuel Cell's Future Gets a Boost," Design News. August 18, 1986, p. 38.
"Zirconium," Machine Design. April 14, 1988, pp. 234-35.
"Zirconium Holds Down Costs of Making Zirconium," Metal Progress. November, 1983, pp. 11-12.
"Adding Strength to Glassy Ceramics," Science News. September 13, 1986, p. 170.
— Alicia Haley and
Blaine Danley
Blaine Danley
Read more: http://www.madehow.com/Volume-1/Zirconium.html#ixzz2dXqMVgkb
source http://www.madehow.com/Volume-1/Zirconium.html
No comments:
Post a Comment